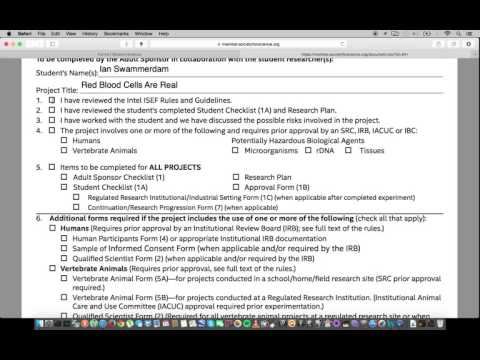
This is a tutorial for the checklist for adult sponsor, Form 1. All students in ninth through 12th grade participating in Slopes F must complete this form with their adult sponsor. An adult sponsor can be anyone, such as a teacher, parent, professor, or any other professional scientist who has experience in the field you're working in. Your adult sponsor should be filling out this form. If you're the adult sponsor, you should have already reviewed your student's Checklist 1a and his or her research plan. You must also be familiar with the regulations that govern potentially hazardous or dangerous research as it may apply to this specific project. To start, put in your student's name and his or her project title. In this example, we're using Ian Swami Damn as your student and "Red Blood Cells are Real" as his or her project title. Once you have completed that, you can review the first five questions. Indicate that you have reviewed the Intel ICF rules and guidelines. Also, indicate that you have reviewed the student's completed Checklist Form 1a, as well as his or her research plan. Indicate that you have worked with the student and that both of you have discussed any potential risks involved in this project. Finally, indicate whether or not this project uses any humans, vertebrate animals, or potentially hazardous biological agents, such as microorganisms, or DNA tissues. If not, you can leave this blank. Finally, make sure that you have all of the necessary information and forms you need to submit this form. Form 1a, your student needs to check that as well. He or she needs to submit a research plan, and he or she needs the approval form 1b. If you're working in a regulated research institution or an industrial setting, you'll also...
Award-winning PDF software





Irb science fair Form: What You Should Know
Form (1D), Regulated Research Institutional / Industrial Setting. Please note, this version of this form is to be used only by IRB approved projects in the United States. A completed version will be returned. International Rules for Human Participant Protection and Data Collection Forms A. Required for research projects in a research organization, where humans are used to test engineering design (or any scientific/engineering idea). B. Required for research projects in a research organization (except where explicitly exempted by the organization) where humans are used to test engineering design or any other scientific/engineering idea. International Rules for Human Participant Protection and Data Collection Forms — Society for Science These forms constitute written documentation of what will occur, or in some cases, has already occurred, in a research project. They are also designed to provide Human Participant Form (2C) (2F) — NET Approval Form (1B) — NET A completed form is required for each student, including all team members. A completed form must be completed by all parties before experiment can take place. Forms (1C), (1D) and (2C) are reviewed by Science Board of the Society for Science. Additional International Rules for Human Participant Protection and Study Form (5A), International Rules for Human Experiments The regulations of the United States Department of Health and Human Services (HHS) Regulated Research Institutional / Industrial Setting, and the Regulated Research Institutional / Industrial Setting (IRS) are the laws required for human subjects in human subjects research. For IRS, the IRB must approve every project, and each group must submit at least one approved form. All forms are to be submitted with the IRB approval form. Forms need to be completed by the supervising adult of any IRB approved project conducted in a research institution, and by all parties who will participate in such project. International Rules for Human Subjects Protection and Study Form (5B), International Rules for Human Experiments The regulations of the United States Department of Health and Human Services (HHS) IRB are the laws required for human subjects in human subjects research. Forms will be received by the Science Board of the Society for Science, where they will be reviewed and approved by Science Board and the IRB.
Online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do Form 12333, steer clear of blunders along with furnish it in a timely manner:
How to complete any Form 12333 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your Form 12333 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your Form 12333 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Irb science fair